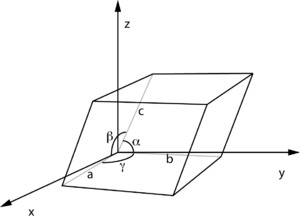
The unit cell is the basic building block of a crystal lattice (whether an atomic crystal or a nanoscale superlattice). Crystalline materials have a periodic structure, with the unit cell being the minimal volume necessary to fully describe the repeating structure. There are a finite number of possible symmetries for the repeating unit cell.
Notation
- Real space:
- Crystal planes:
- (hkl) denotes a plane of the crystal structure (and repetitions of that plane, with the given spacing). In cubic systems (but not others), the normal to the plane is [hkl]
- {hkl} denotes the set of all planes that are equivalent to (hkl) by the symmetry of the lattice
- Crystal directions:
- [hkl] denotes a direction of a vector (in the basis of the direct lattice vectors)
 denotes the set of all directions that are equivalent to [hkl] by symmetry (e.g. in cubic system <100> means [100, [010], [001], [-100], [0-10], [00-1])
denotes the set of all directions that are equivalent to [hkl] by symmetry (e.g. in cubic system <100> means [100, [010], [001], [-100], [0-10], [00-1])
- hkl denotes a diffracting plane
- Reciprocal space:
- Reciprocal planes:
- [hkl] denotes a plane
 denotes the set of all planes that are equivalent to [hkl]
denotes the set of all planes that are equivalent to [hkl]
- Reciprocal directions:
- (hkl) denotes a particular direction (normal to plane (hkl) in real space)
- {hkl} denotes the set of all directions that are equivalent to (hkl)
- hkl denotes an indexed reflection
Math
Vectors

Relations



Volume

If a, b, and c are the parallelepiped edge lengths, and α, β, and γ are the internal angles between the edges, the volume is

The volume of a unit cell with all edge-length equal to unity is:

Angles
 is the angle between
is the angle between  and
and 
 is the angle between
is the angle between  and
and 
 is the angle between
is the angle between  and
and 
Reciprocal Vectors

Vector components
Generally:

With components:

Examples
Cubic
Since  ,
,  , and:
, and:

And in reciprocal-space:

So:

Hexagonal
Since  and
and  ,
,  , and:
, and:

And in reciprocal-space:

So: