|
|
| Line 1: |
Line 1: |
| − | The diamond lattice, which consists of tetrahedrally-arranged atoms/particles, has [[Lattice:Simple Cubic|simple cubic]] symmetry. | + | The diamond [[lattice]], which consists of tetrahedrally-arranged atoms/particles, has [[Lattice:Simple Cubic|simple cubic]] [[symmetry]]. |
| | | | |
| | [[Image:Diamond00.png]] | | [[Image:Diamond00.png]] |
| Line 5: |
Line 5: |
| | | | |
| | | | |
| − | Along an appropriate axis, the lattice has six-fold symmetry (hexagonal). | + | Along an appropriate axis, the lattice has six-fold symmetry ([[hexagonal]]). |
| | | | |
| | ==Canonical Diamond== | | ==Canonical Diamond== |
Revision as of 17:27, 13 November 2016
The diamond lattice, which consists of tetrahedrally-arranged atoms/particles, has simple cubic symmetry.
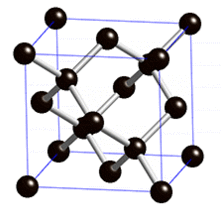
Along an appropriate axis, the lattice has six-fold symmetry (hexagonal).
Canonical Diamond
A canonical diamond lattice (single atom/particle type arranged as shown above) has symmetry Fd3m. The atoms occupy the positions (0,0,0;1/4,1/4,1/4)+face-centering.
Symmetry
- Crystal Family: Cubic
- Crystal System: Cubic
- Bravais Lattice: F (fcc)
- Crystal class: Hexoctahedral
- Point Group: d3m
- Space Group: Fd3m
- Particles per unit cell:

- Volume of unit cell:

- Dimensionality:

- Projected d-dimensional volume:

- Solid angle:

- Nearest-neighbor distance:

- Assuming spherical particles of radius R:
- Particle volume fraction:

- Maximum volume fraction:
 when
when 
Structure
The lattice may be thought of as two interpenetrating FCC lattices.
Particle Positions
There are 18 positions. In total there are 8 particles in the unit cell:









Examples
Elemental
- 6. Carbon (C) (a = 3.567 Å)
- 14. Silicon (Si) (a = 5.431 Å)
- 32. Germanium (Ge) (a = 5.657 Å)
- 50. Gray Tin (Sn) (a = 6.491 Å)
Atomic
Nano
- Nanoparticle superlattice
- Liu, W.; Tagawa, M.; Xin, H.L.; Wang, T.; Emamy, H. Li, H.; Yager, K.G.; Starr, F.W.; Tkachenko, A.V.; Gang, O. Diamond family of nanoparticle superlattices Science 2016, 351, 582–586. doi: 10.1126/science.aad2080
Diamond-like Two-particle
Also known as zincblende. This is effectively

Examples
Atomics
Double-filled Diamond-like Two-particle
The diamond lattice includes 8 "tetrahedral holes", with only 4 occupied in a 'normal' diamond structure. A two-particle lattice can be formed by filling all 8 internal holes with the 2nd particle-type, in which case the particles exist in a 1:2 ratio. This is often called a CaF2 lattice.

Particle Positions
There are 22 positions. In total there are 12 particles in the unit cell:














Neighbors
There are 12 particles in the unit cell: 4 of type A, and 8 of type B. The type A particles each connect to 8 particles of type B. The type B particles each connect (tetrahedrally) to 4 particles of type A.

The lattice may also be thought of as having cubes of type B with a connected type A particle at the center. However the extended lattice is not BCC-like, since only every second "BCC cube" has a central particle of type A.

Examples
Atomics
Double Inter-penetrating Diamond Two-Particle
This lattice is formed from two diamond lattices that inter-penetrate. It is often called the NaTl lattice.
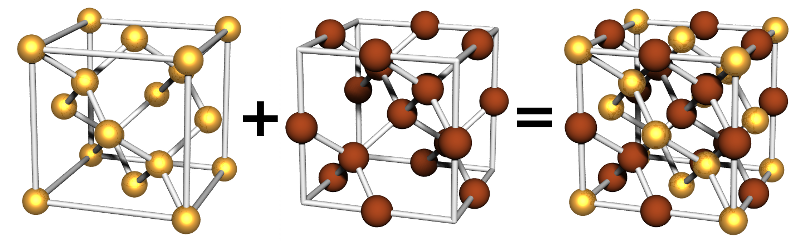
Particle Positions
There are 36 positions. In total there are 16 particles in the unit cell, 8 of each type.
Particle Type A









Particle Type B










Examples
Atomics
- Sodium Thallium crystal (NaTl)
Nanoparticles
Cristobalite
Cristobalite is a diamond-like lattice formed in silicon dioxide (SiO2). The Si atoms bond tetradrally, each to four O atoms. Each O atom is bonded to 2 Si atoms, effectively acting as a bridge.
Structure
Can be thought of as a diamond lattice, where the tetrahedral species sit on the positions of the canonical diamond sites, and the two-bonded species sites at the midway point of each bond.



The tetrahedral species sit on the 14 "FCC" sites, plus 4 "internal" diamond sites. The two-bonded species sit on the 16 internal bonds in the unit cell. The tetrahedral species sit at a distance from one another of:
- Next-nearest-neighbor distance:

The different species thus have a distance of half that:
- Nearest-neighbor distance:

Particle Positions
There are 34 positions (24 in unit cell)
Particle Type A (bond tetrahedrally)
There are 18 positions. In total there are 8 particles in the unit cell:









Particle Type B (two-fold bonded)
There are 16 positions (all 16 in the unit cell):




















Examples
Atomics
See Also




























































