Difference between revisions of "Neutron scattering lengths"
KevinYager (talk | contribs) (→Neutron Scattering Length Density) |
KevinYager (talk | contribs) (→Neutron Scattering Length Density) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | For | + | For [[neutron]]s, the ''b<sub>i</sub>'' in the [[SLD]] equation are the [[neutron scattering lengths]] for the atoms in question. |
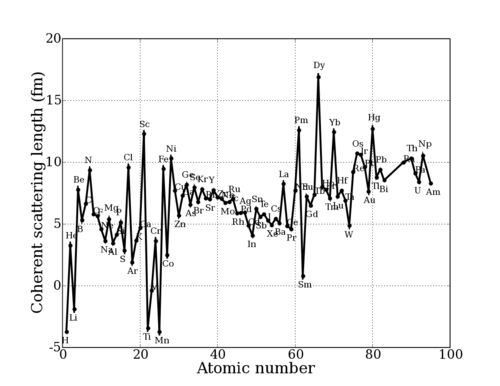
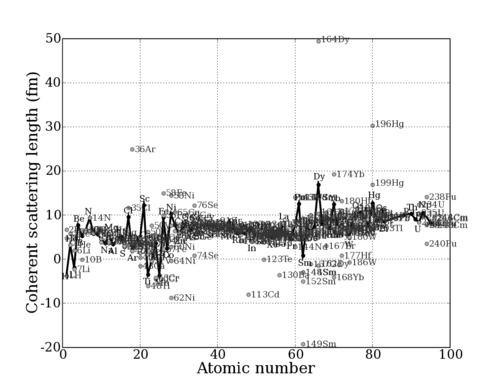
This value depends sensitively on the details of the nuclear interaction, and thus on the nuclear properties (spin, energy levels, etc.) of any particular nuclide (isotopic nucleus). Thus, the scattering length varies seemingly irregularly throughout the periodic table. (Of course, [[Origin of neutron scattering lengths|there is an underlying reason]] for this.) This can be seen in the graphs below. | This value depends sensitively on the details of the nuclear interaction, and thus on the nuclear properties (spin, energy levels, etc.) of any particular nuclide (isotopic nucleus). Thus, the scattering length varies seemingly irregularly throughout the periodic table. (Of course, [[Origin of neutron scattering lengths|there is an underlying reason]] for this.) This can be seen in the graphs below. | ||
| Line 14: | Line 14: | ||
[[Image:SLD graph-water mixing.png|500px]] | [[Image:SLD graph-water mixing.png|500px]] | ||
| − | It is also noteworthy to compare this variation with the corresponding variation for x-ray interactions. Because x-ray interactions are governed by electron clouds, they increase roughly with atomic number (''Z''). This difference in behavior between x-rays and neutrons make the techniques highly complementary. For one thing, x-rays scattering most efficiently from high-''Z'' materials, whereas neutrons can be used on lighter atoms (e.g. soft matter containing mostly C and H). Studying a single system with both techniques also provides multiple contrast conditions, which makes data interpretation more robust. | + | It is also noteworthy to compare this variation with the [[Atomic scattering factors|corresponding variation]] for [[x-ray]] interactions. Because x-ray interactions are governed by electron clouds, they increase roughly with atomic number (''Z''). This difference in behavior between x-rays and neutrons make the techniques highly complementary. For one thing, x-rays scattering most efficiently from high-''Z'' materials, whereas neutrons can be used on lighter atoms (e.g. soft matter containing mostly C and H). Studying a single system with both techniques also provides multiple contrast conditions, which makes data interpretation more robust. |
==See Also== | ==See Also== | ||
Latest revision as of 12:22, 20 January 2015
For neutrons, the bi in the SLD equation are the neutron scattering lengths for the atoms in question.
This value depends sensitively on the details of the nuclear interaction, and thus on the nuclear properties (spin, energy levels, etc.) of any particular nuclide (isotopic nucleus). Thus, the scattering length varies seemingly irregularly throughout the periodic table. (Of course, there is an underlying reason for this.) This can be seen in the graphs below.
Contents
Neutron scattering length for Elements
Neutron scattering length for Isotopes
Neutron Scattering Length Density
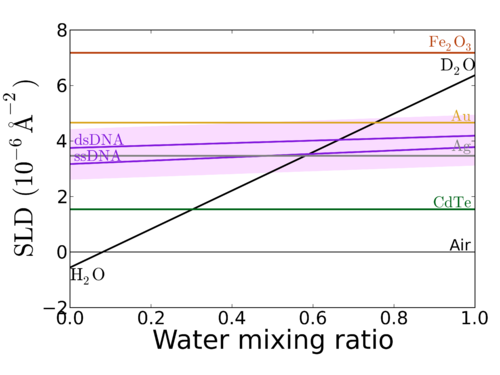
The neutron scattering lengths directly control the Scattering Length Density (SLD) of materials; i.e. the strength of neutron scattering from a given material. The wide variation of SLD for materials makes neutrons a uniquely powerful structural probe. On the one hand, one can vary the contrast condition simply by changing one isotope for another. For example, selective deuteration (of a material or a solvent) can be used to enhance a particular contrast, or to 'contrast match' a particular structure, thereby making it invisible to the neutron beam (and highlighting other structures). One can also attempt to, e.g., substitute one ion for an isovalent ion with a radically different SLD; or to perform the same experiment twice, with the substrate (or even just a layer in the substrate) swapped. The different contrast conditions allow for more robust data fitting (since a solution consistent with both datasets can resolve fitting ambiguities).
A common 'trick' in neutron experiments is to vary the ratio of H2O to D2O in the solvent; which allows one to vary the ambient SLD across a very wide range:
It is also noteworthy to compare this variation with the corresponding variation for x-ray interactions. Because x-ray interactions are governed by electron clouds, they increase roughly with atomic number (Z). This difference in behavior between x-rays and neutrons make the techniques highly complementary. For one thing, x-rays scattering most efficiently from high-Z materials, whereas neutrons can be used on lighter atoms (e.g. soft matter containing mostly C and H). Studying a single system with both techniques also provides multiple contrast conditions, which makes data interpretation more robust.
See Also
- Neutron data booklet: Tabulates fundamental properties of neutron interactions with matter; also provides useful equations.
- NIST: Periodic table of neutron scattering lengths and cross sections
- SLD calculator: NIST Center for Neutron Research calculator for predicting the neutron Scattering Length Density (SLD) for materials.