|
|
| (3 intermediate revisions by the same user not shown) |
| Line 48: |
Line 48: |
| | For a particle-particle bond-length of <math>l</math>: | | For a particle-particle bond-length of <math>l</math>: |
| | :<math>a=\frac{2\sqrt{6}}{3}l</math> | | :<math>a=\frac{2\sqrt{6}}{3}l</math> |
| | + | :<math>b=a=\frac{2\sqrt{6}}{3}l</math> |
| | :<math>c=\frac{8}{3}l</math> | | :<math>c=\frac{8}{3}l</math> |
| | :<math>\frac{a}{c}=\frac{\sqrt{6}}{4}\approx 0.61237</math> | | :<math>\frac{a}{c}=\frac{\sqrt{6}}{4}\approx 0.61237</math> |
| | :<math>\frac{c}{a}=\frac{4}{\sqrt{6}}\approx 1.63299</math> | | :<math>\frac{c}{a}=\frac{4}{\sqrt{6}}\approx 1.63299</math> |
| | + | |
| | + | ====Absolute (in terms of particle-particle distance)==== |
| | + | * <math> 4 \, \mathrm{bottom\,\, layer}: \, \frac{1}{12} + \frac{1}{6} + \frac{1}{12} + \frac{1}{6} = \frac{1}{2}</math> |
| | + | ** <math>\left(0,0,0\right),\left(\frac{2\sqrt{6}}{3}l,0,0 \right),\left(\frac{\sqrt{6}}{3}l,\sqrt{2}l,0\right),\left(\sqrt{6}l,\sqrt{2}l,0\right)</math> |
| | + | * <math> 4 \, \mathrm{mid\,\, layer}: \, \frac{1}{6} + \frac{1}{3} + \frac{1}{6} + \frac{1}{3} = 1</math> |
| | + | ** <math>\left(0,0,\frac{5}{3}l\right),\left(\frac{2\sqrt{6}}{3}l,0,\frac{5}{3}l \right),\left(\frac{\sqrt{6}}{3}l,\sqrt{2}l,\frac{5}{3}l\right),\left(\sqrt{6}l,\sqrt{2}l,\frac{5}{3}l\right)</math> |
| | + | * <math> 2 \, \mathrm{internal\,\, strut}: \, 1 + 1 = 2</math> |
| | + | ** <math>\left(\frac{\sqrt{6}}{3}l,\frac{\sqrt{2}}{3}l,\frac{1}{3}l\right),\left(\frac{\sqrt{6}}{3}l,\frac{\sqrt{2}}{3}l,\frac{4}{3}l\right)</math> |
| | + | * <math> 4 \, \mathrm{top\,\, layer}: \, \frac{1}{12} + \frac{1}{6} + \frac{1}{12} + \frac{1}{6} = \frac{1}{2}</math> |
| | + | ** <math>\left(0,0,\frac{8}{3}l\right),\left(\frac{2\sqrt{6}}{3}l,0,\frac{8}{3}l \right),\left(\frac{\sqrt{6}}{3}l,\sqrt{2}l,\frac{8}{3}l\right),\left(\sqrt{6}l,\sqrt{2}l,\frac{8}{3}l\right)</math> |
| | | | |
| | ===Examples=== | | ===Examples=== |
| Line 57: |
Line 68: |
| | | | |
| | ==Alternating Hexagonal Diamond== | | ==Alternating Hexagonal Diamond== |
| − | This is the [http://en.wikipedia.org/wiki/Wurtzite_%28crystal_structure%29 Wurtzite] crystal structure, a hexgonal unit cell with alternating species. | + | This is the [[Lattice:Wurtzite|Wurtzite]] crystal structure, a hexagonal unit cell with alternating species. |
| | ===Examples=== | | ===Examples=== |
| | ====Atomics==== | | ====Atomics==== |
| | * [http://en.wikipedia.org/wiki/Wurtzite Wurtzite] (Zn,Fe)S (a = b = 3.82 Å, c = 6.26 Å) | | * [http://en.wikipedia.org/wiki/Wurtzite Wurtzite] (Zn,Fe)S (a = b = 3.82 Å, c = 6.26 Å) |
| − |
| |
| | | | |
| | ==Along Connections== | | ==Along Connections== |
| Line 88: |
Line 98: |
| | | | |
| | ==See Also== | | ==See Also== |
| − | * [http://en.wikipedia.org/wiki/Lonsdaleite Lonsdaleite (Wikipedia)] | + | * [http://en.wikipedia.org/wiki/Lonsdaleite Wikipedia: Lonsdaleite] |
| | * [[Lattice:Diamond| Cubic Diamond Lattice]] | | * [[Lattice:Diamond| Cubic Diamond Lattice]] |
The hexagonal diamond lattice is an arrangement of tetrahedrally-bonded elements, within a hexagonal unit cell. Whereas conventional diamond (a.k.a. cubic diamond) exists within a cubic unit cell, hexagonal diamond exists within a hexagonal unit cell. In both cases, elements are bonded tetrahdrally. However, in cubic diamond, the six-membered rings are all in the chair conformation, whereas in hexagonal diamond, some six-membered rings are in the boat conformation.
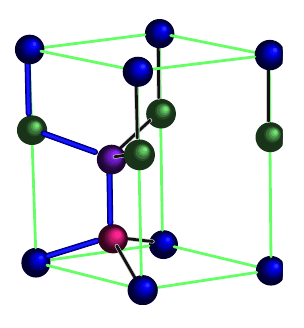
The four distinct positions in the unit cell are each given a different color. From: "Structural and vibrational properties of the 6H diamond: First-principles study"
doi: 10.1016/j.diamond.2006.03.013
Canonical Hexagonal Diamond
A canonical hexagonal diamond lattice (single atom/particle type arranged as shown above) has symmetry Fd3m.
Symmetry
- Crystal Family: Hexagonal
- Crystal System: Hexagonal
- Bravais Lattice: hexagonal
- Crystal class: Hexoctahedral
- Space Group: P63/mmc
- Particles per unit cell:

- Volume of unit cell:

- Dimensionality:

Structure
TBD
Particle Positions
There are 14 positions. In total there are 4 particles in the unit cell.
Fractional
Positions are given in terms of fractional coordinates relative to the unit-cell edge-vectors:











Absolute








Distances
For a particle-particle bond-length of  :
:





Absolute (in terms of particle-particle distance)








Examples
Atomics
- Lonsdaleite form of carbon (C), also known as hexagonal diamond, 2H diamond, or 'sp3 diamond' (a = 2.51 Å, c = 4.12 Å)
Alternating Hexagonal Diamond
This is the Wurtzite crystal structure, a hexagonal unit cell with alternating species.
Examples
Atomics
- Wurtzite (Zn,Fe)S (a = b = 3.82 Å, c = 6.26 Å)
Along Connections
This lattice can be thought of as the hexagonal-dimaond analog of the cubic-diamond cristobalite. Here, a four-bonded species occupies all the sites of the canonical hexagonal diamond lattice, and a two-bonded species sits along each of the connections between these tetrahedral sites.
Particle Positions
Particle Type A (bond tetrahedrally)
These are the same positions as the canonical hexagonal diamond.
Particle Type B (two-fold bonded)
There are 11 positions. In total there are 8 particles of this type in the unit cell.
Fractional
Positions are given in terms of fractional coordinates relative to the unit-cell edge-vectors:











See Also




































