Difference between revisions of "Material:P3HT"
KevinYager (talk | contribs) |
KevinYager (talk | contribs) |
||
| Line 5: | Line 5: | ||
P3HT typically organizes into a semi-crystalline state, with an idealized [[unit cell]] as shown below: | P3HT typically organizes into a semi-crystalline state, with an idealized [[unit cell]] as shown below: | ||
[[Image:Cell3-main2.png|thumb|center|300px|Cartoon of an idealized P3HT [[unit cell]].]] | [[Image:Cell3-main2.png|thumb|center|300px|Cartoon of an idealized P3HT [[unit cell]].]] | ||
| + | * The lamellar stacking has a spacing of ~1.6 nm, [[Q value|giving rise]] to a peak (100) at ''q'' ~0.4 Å<sup>−1</sup>. | ||
| + | * The aromatic (''π''-''π'') stacking has a spacing of ~0.39 nm, [[Q value|giving rise]] to a peak (010) at ''q'' ~1.6 Å<sup>−1</sup>. | ||
| + | * The spacing along the ring direction is too disordered to give a well-defined 001 peak. | ||
| + | |||
Refer to [[Example:P3HT_orientation_analysis|P3HT orientation]] for details about the organization of P3HT. | Refer to [[Example:P3HT_orientation_analysis|P3HT orientation]] for details about the organization of P3HT. | ||
==Scattering== | ==Scattering== | ||
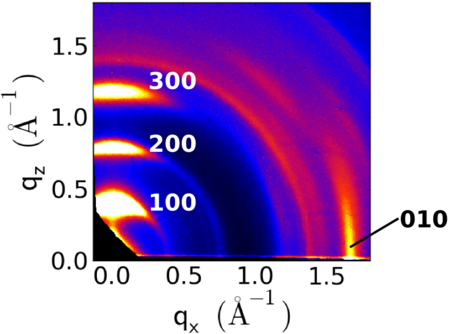
| − | + | On flat substrates, P3HT typically organizes into an ''edge-on'' orientation, wherein the lamellar stacking direction is along the film normal (leading to the 100 peak being along ''q<sub>z</sub>''), and the aromatic stacking then being in-plane (010 peak along ''q<sub>r</sub>''). | |
| + | [[Image:P3ht-generic giwaxs02.png|450px|center]] | ||
==Properties== | ==Properties== | ||
Revision as of 15:20, 18 June 2014
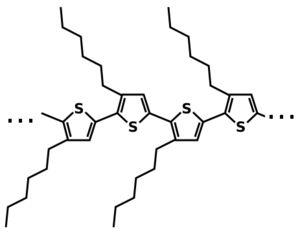
Poly(3-hexylthiophene) (P3HT) is a polymer with chemical formula (C10H14S)n. It is a polythiophene with a short alkyl group on each repeat unit. It is noteworthy since is a seminconducting polymer; it can conduct positive charges (holes). It is a common material for studies of organic electronics (e.g. FETs) and for organic photovoltaics (OPV).
Contents
Organization
P3HT typically organizes into a semi-crystalline state, with an idealized unit cell as shown below:
- The lamellar stacking has a spacing of ~1.6 nm, giving rise to a peak (100) at q ~0.4 Å−1.
- The aromatic (π-π) stacking has a spacing of ~0.39 nm, giving rise to a peak (010) at q ~1.6 Å−1.
- The spacing along the ring direction is too disordered to give a well-defined 001 peak.
Refer to P3HT orientation for details about the organization of P3HT.
Scattering
On flat substrates, P3HT typically organizes into an edge-on orientation, wherein the lamellar stacking direction is along the film normal (leading to the 100 peak being along qz), and the aromatic stacking then being in-plane (010 peak along qr).
Properties
- Density: ~1.33 g/cm3
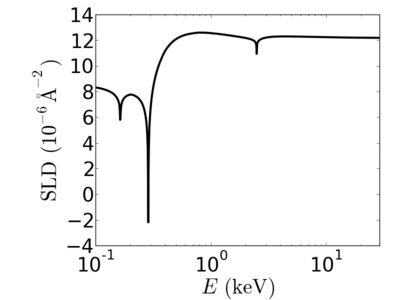
- Neutron SLD: 0.817×10−6 Å−2
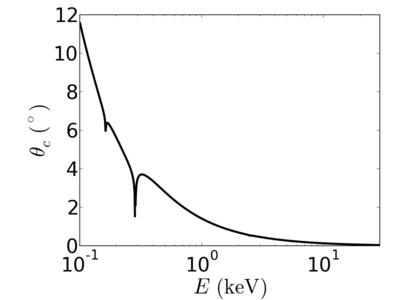
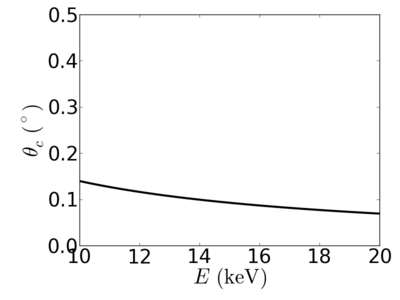
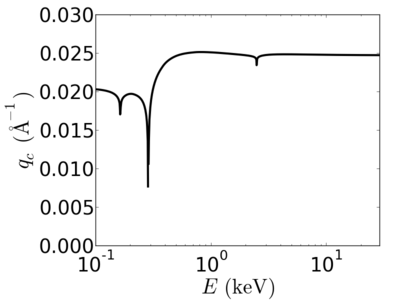
| Material | density (g/cm3) | X-ray energy (keV) | X-ray wavelength (Å) | critical angle (°) | qc (Å−1) | SLD (10−6Å−2) |
|---|---|---|---|---|---|---|
| P3HT | 1.33 | 2.0 | 6.20 | 0.700 | 0.0248 | 12.20 |
| 4.0 | 3.10 | 0.352 | 0.0249 | 12.33 | ||
| 8.0 | 1.55 | 0.176 | 0.0249 | 12.29 | ||
| 12.0 | 1.03 | 0.117 | 0.0248 | 12.25 | ||
| 16.0 | 0.77 | 0.088 | 0.0248 | 12.24 | ||
| 24.0 | 0.52 | 0.058 | 0.0248 | 12.2 |