Example of the
BCC unit cell.
The unit cell is the basic building block of a crystal lattice (whether an atomic crystal or a nanoscale superlattice). Crystalline materials have a periodic structure, with the unit cell being the minimal volume necessary to fully describe the repeating structure. There are a finite number of possible symmetries for the repeating unit cell.
A unit cell can be defined by three vectors that lie along the edges of the enclosing parallelepped. We denote the vectors as  ,
,  , and
, and  ; alternately the unit cell can be described by the lengths of these vectors (
; alternately the unit cell can be described by the lengths of these vectors ( ,
,  ,
,  ), and the angles between them:
), and the angles between them:
 , the angle between
, the angle between  and
and 
 , the angle between
, the angle between  and
and 
 , the angle between
, the angle between  and
and 
Mathematical description
Vectors

Relations



Volume

If a, b, and c are the parallelepiped edge lengths, and α, β, and γ are the internal angles between the edges, the volume is

The volume of a unit cell with all edge-length equal to unity is:

Angles
 is the angle between
is the angle between  and
and 
 is the angle between
is the angle between  and
and 
 is the angle between
is the angle between  and
and 
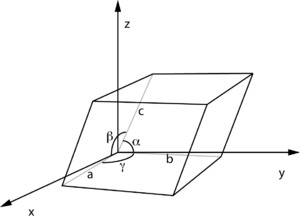
Unit cell definition using parallelepiped with lengths
a,
b,
c and angles between the sides given by α,β,γ (from Wikipedia
fractional coordinates).
Reciprocal vectors
The repeating structure of a unit cell creates peaks in reciprocal space. In particular, we observe maxima (constructive interference) when:

Where  ,
,  , and
, and  are integers. We define reciprocal-space vectors:
are integers. We define reciprocal-space vectors:

And we can then express the momentum transfer ( ) in terms of these reciprocal vectors:
) in terms of these reciprocal vectors:

Combining with the three Laue equations yields:

Where  is a vector that defines the position of Bragg reflection
is a vector that defines the position of Bragg reflection  for the reciprocal-lattice.
for the reciprocal-lattice.
Examples
Cubic
Since  ,
,  , and:
, and:

And in reciprocal-space:

So:

And:

Hexagonal
Since  and
and  ,
,  , and:
, and:

And in reciprocal-space:

So:

See Also







































